Hydrated Transition Metal Ions Typically Produce Solutions That Are
Cl 2 l iron III compounds are usually orangebrown e. The positive ions which are usually smaller than the negative ions show this effect to a greater extent.

Transition Metals Chemical Reactions Physical Properties Uses Alloys Balanced Symbol Word Equations Copper Iron Zinc Titanium Steel Chromium Brass Superconductors Gcse Chemistry Revision Notes Igcse O Level Ks4 Science
This solution mixture was precipitated by gradual addition of NH3 solution 25 wt till pH reached a value of 10.

. Hydrated transition metal ions typically produce solutions that are _____ a acidic. In cases where waters of hydration are coordinated directly to the metal ions heating usually does not produce the anhydrous salt but rather an oxohalide or an impure product. The 3 ions are noticeably more acidic.
If they are present a. The presence of transition metal ions in a solution can be tested by adding sodium hydroxide solution. This helps explain for example why rust iron oxide is an orange colour and why the.
A at the bottom left b in the top left c in the transition metals d at the bottom. The acidity of MH2O63 is greater than that of MH2O62 in terms of the greater polarising power. Up to 24 cash back ions are added to a solution containing hydrated transition-metal ions.
The pure metal oxides such as CuO NiO and CO 3 O 4 were prepared with the CP method. Hydrated metal ions like CrH 2 O 6 3 are capable of donating protons to water and acting as weak acids. This graphic looks at the colours of transition metal ions when they are in aqueous solution in water and also looks at the reason why we see coloured compounds and complexes for transition metals.
It is easy to understand why aqueous solutions of HCl or CH 3 CO 2 H are acidic. Highly charged metal ions such as A l 3 produce acidic solutions. Second the highest ox states like CrO4-- and MnO4-.
3 transition metals are moderately acidic. C neutral d strongly basic. The colour of light absorbed by the complexed ion is related to electronic energy changes in the structure of the complex.
One would expect a metal complex with no d-eletron to be colorless as well. Cu aq D-a green Ni aq DO colorless Ag aq blue Co aq co pink Do you know the answer. CuNO 3 23H 2 O NiNO 3 26H 2 O and CoNO 3 26H 2 O were dissolved in deionized hot water and the solution was heated up to 65 C.
The following equilibria happen in aqueous solutions of metal ions. Transition Metal Ions. Most of the transition metal compounds ionic as well as covalent are coloured both in solid state in an aqueous state.
The hydrated HgII ion is also noticeably. 2 transition metal ions are weakly acidic. As an example iron commonly forms two different ions.
Unsure No idea Think so. As previously mentioned transition metal complexes have bright colours. Alkaline earth metal ions are only slightly acidic.
Copper II sulfate Cu. The solvation number n determined by a variety of experimental methods is 4 for Li and Be 2 and 6 for elements in periods 3 and 4 of the periodic table. This results in hydrated cations which may have considerable stability especially for the di- and tri-valent transition metal ions.
This is insoluble so it appears as a solid called a. It can sometimes lose two electrons to form the ceFe2 ion while at other times it loses three electrons to form the. Lanthanide and actinide aqua ions have a solvation number of 8 or 9.
Once a soluble ionic solid like NaCl is placed into water the polar solvent begins working. Transition-Metal Ions as Brønsted Acids. Cu2 ions produce a blue precipitate of CuOH 2 Fe2 ions produce a greygreen precipitate of FeOH 2 Fe3 ions produce an orangebrown precipitate of FeOH 3.
Most hydrated ions with a charge of 3 like Al 3 and Fe 3 behave similarly and are about as strong as acetic acid. CoCl26H2O -- CoCl2 6H2O The mass percentage of water in a hydrate can usually be determined by heating a known amount of the hydrate until dehydration occurs and the waters have been completely driven off. Most transition metals differ from the metals of Groups 1 2 and 13 in that they are capable of forming more than one cation with different ionic charges.
Metal ions with larger charges and smaller radii are stronger lewis acids hence Alkali metal ions show essentially no acidity. L iron II compounds are usually green e. When aqueous solutions of various salts are concentrated.
The equilibria lead to generation of acidic solutions with M3 ions and very weakly acidic solutions with M2 ions. Complexes that contain metal ions of d10 electron configuration are usually colorless. Hexaaqua complexes are the most common type of metal cations in aqueous solutions.
Generally the elementsions having unpaired electrons produce the coloured compound. Match the hydrated transition metal ions with their color aqueous solution. Main group metal complexes and compounds are colourless.
This is typically the case when the cation is small andor highly charged or is a transition metal ion. We cant attribute the acidity of these solutions to the Cl - or NO 3- ions because these ions are weak bases. A metal ion in aqueous solution or aqua ion is a cation dissolved in water of chemical formula M H 2 O n z.
Colour and transition metals Most transition metals form coloured compounds. The following data for the pH of 01 M solutions of transition-metal ions are a bit harder to explain. A final complication in dealing with aqueous solutions of transition-metal complexes is their acid-base behavior.
Chemistry questions and answers. Glancing at a periodic table where do you expect to find elements that are good oxidizing agents. Iron III oxide Fe 2 O 3 when hydrated this is rust l copper II compounds are blue e.
A typical transition metal has more than one possible oxidation state because it has a partially filled d orbital. In the competitive adsorption study for the metals ions erionite was more effective in the adsorption process revealing a selectivity of. Colours of Transition Metal Ions in Aqueous Solution.
Examples are CuPPh 34 and ZnH 2O 6 2. However a few of such complexes are strongly colored for example MnO 4-or Cr 2O7 2-. Iron II chloride Fe.
When transition metals bond to one more neutral or negatively charged nonmetal species they form what are called transition metal complexesAnother way to look at a complex ion is as a chemical species with a metal ion at the center and other ions. Answer 1 of 2. PbIINiIIZnIICdII and this order can be.
W Drag statements on the right to match the left. As the name suggests they have six water molecules as ligands. - The water bound in hydrates can often be removed at low temperatures about 120C in most cases to produce water and the anhydrous an- without hydro-water salt.
Not only the chloride ions but the other halide ions are liable to complex and the same is true of species like NH3 and CN. The positive side of waters dipole moment face the Cl-ions creating a spherical. These ligands differ quite a lot in their affinity for a particular metal ion but the rules governing this situation are not simple.
Several things are happening First the higher oxidation states usually have more insoluble hydroxides or oxides so increasing OH- removes ions from solution and forces reaction to higher ox state.

Lesson Explainer Physical Properties Of Transition Metals Nagwa

Antibacterial Mechanisms Of Metal Ions And Nanoparticles The Central Download Scientific Diagram

Transition Metal Ions All Transition Metals Lose Electrons When They React And So Form Positive Ions Some Transition Metals Only Make One Type Of Ion Ppt Download

Solved Hydrated Transition Metal Ions Produce Solutions That Chegg Com
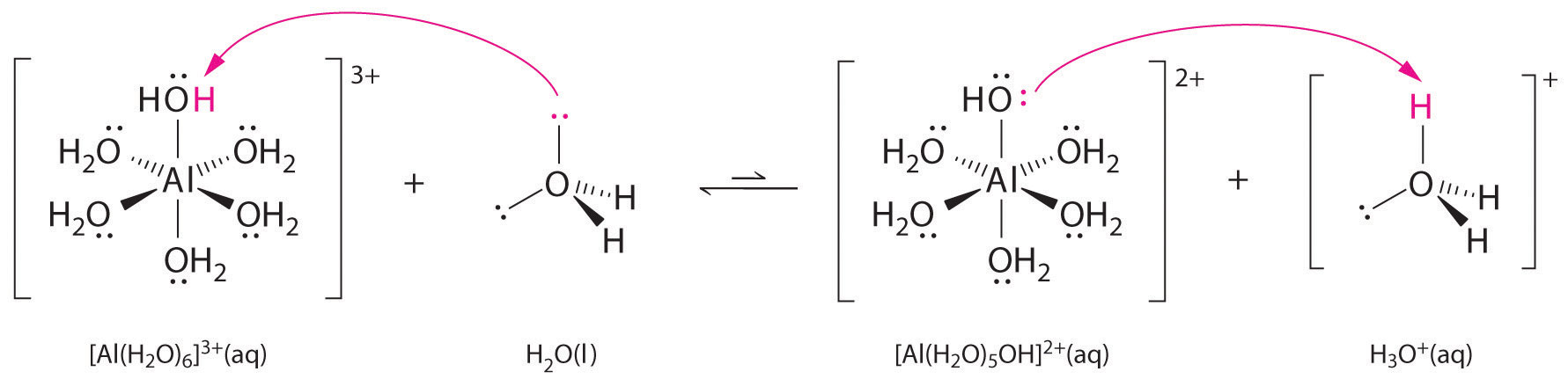
6 3 8 High Charge To Size Ratio Metal Ions Act As Bronsted Acids In Water Chemistry Libretexts

Hydrated Salts Transition Metals Hexa Aqua Complex Ions Relative Acidity Of Hexaaqua Ions Salt Hydrolysis Why Can Metal Ions Be Acidic A Level Gce As A2 Ib A Level Inorganic Chemistry Revision Notes
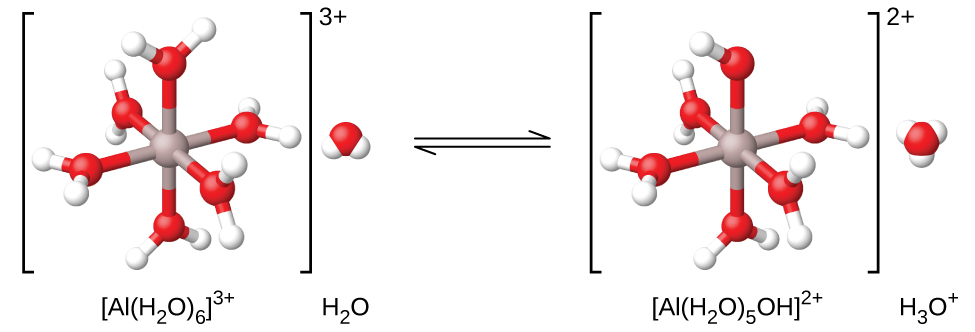
14 4 Hydrolysis Of Salt Solutions Chemistry

Solved Online Version Background An Intriguing Feature Of Chegg Com

Reactions Of Some Transition Metal Ions Cobalt Knockhardy
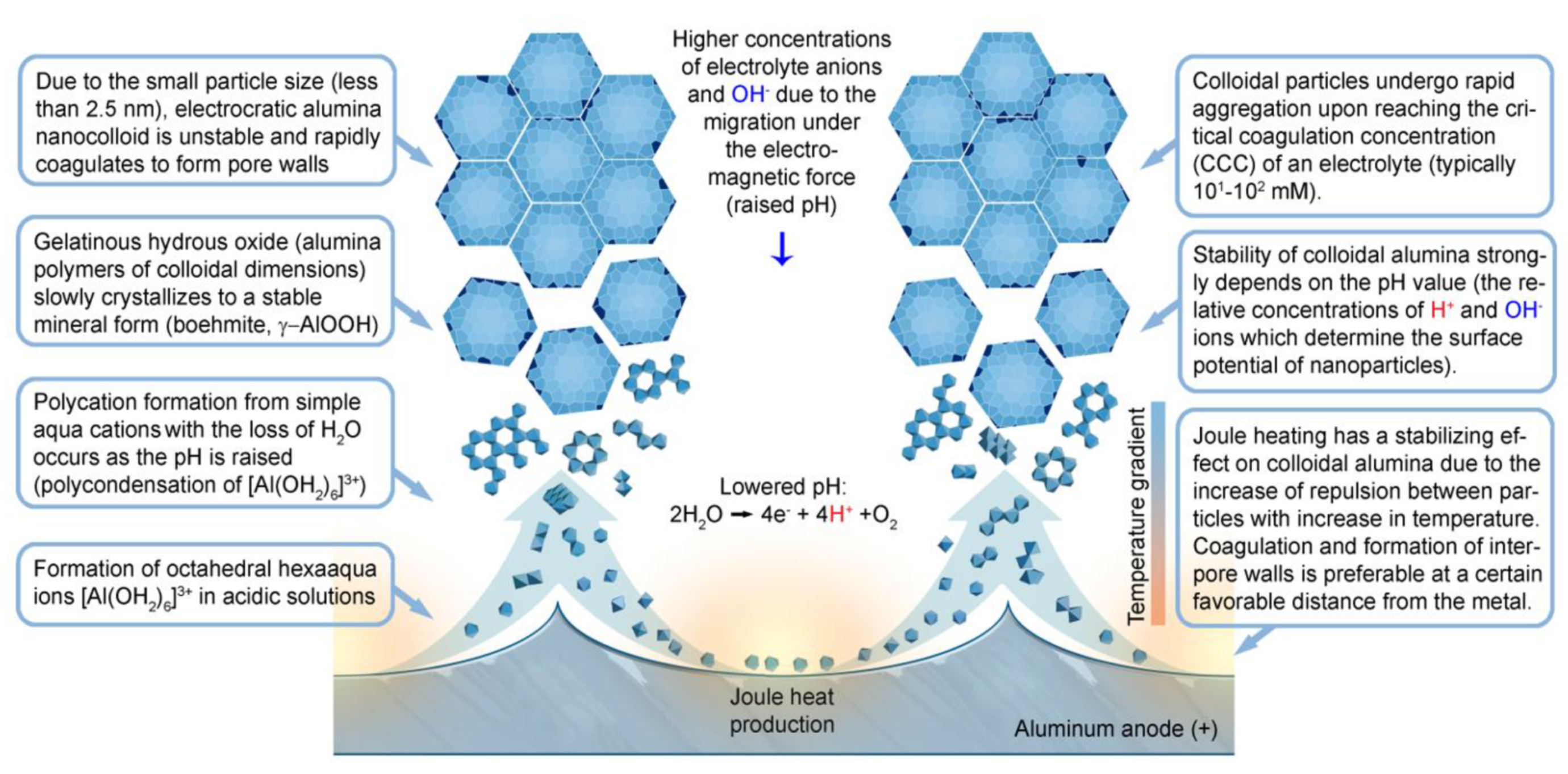
Nanomaterials Free Full Text Conceptual Progress For Explaining And Predicting Self Organization On Anodized Aluminum Surfaces Html

Transition Metaltransition Metaltransition Metaltransition Metalchemistry Ppt Download
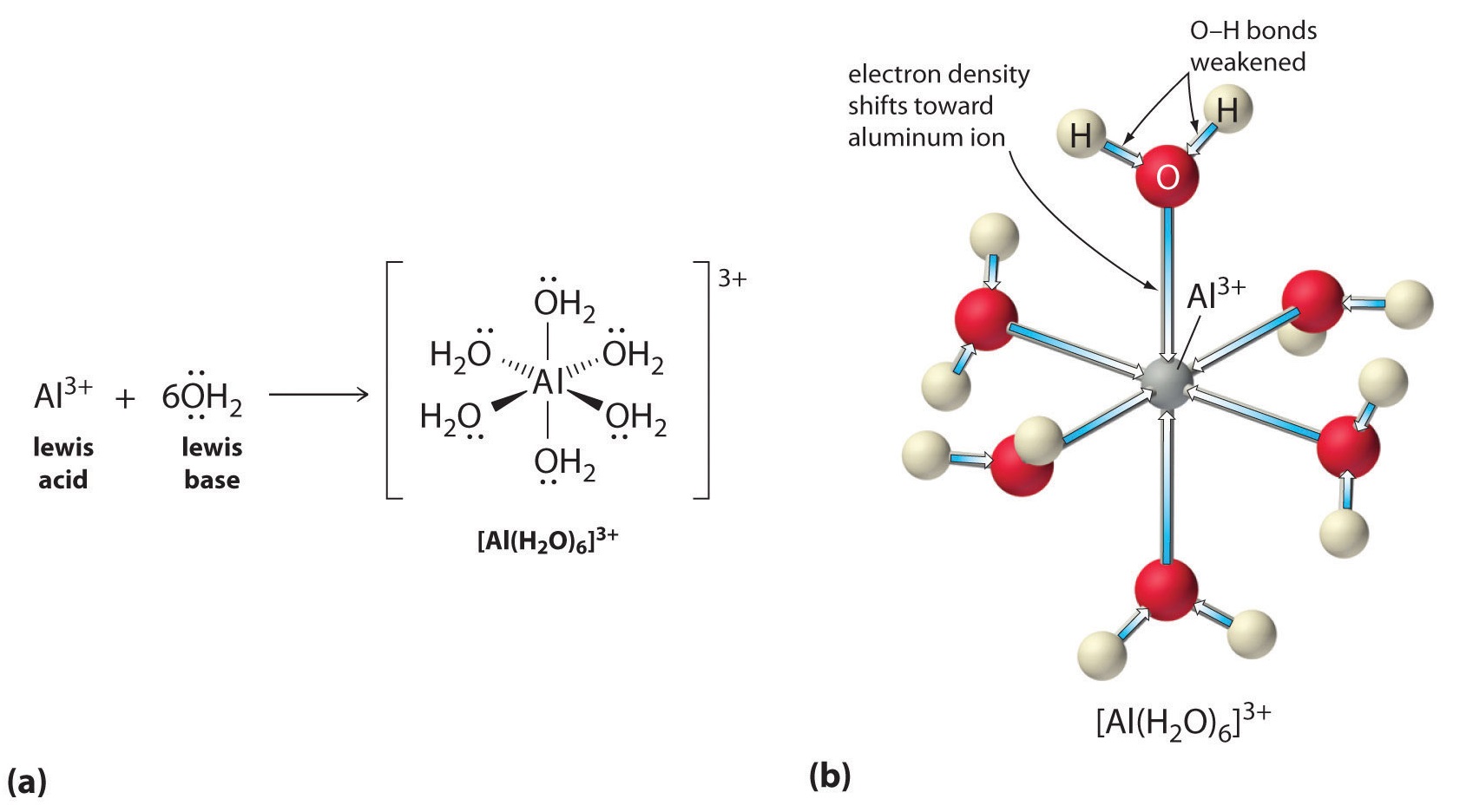
6 3 8 High Charge To Size Ratio Metal Ions Act As Bronsted Acids In Water Chemistry Libretexts

Solved Hydrated Transition Metal Ions Typically Produce Chegg Com

A Hydroxyquinoline Based Unnatural Amino Acid For The Design Of Novel Artificial Metalloenzymes Drienovska 2020 Chembiochem Wiley Online Library

Metals Free Full Text Precipitation And Crystallization Used In The Production Of Metal Salts For Li Ion Battery Materials A Review Html

Iron Transition Metal Chemistry Iron Ii Fe2 Iron Iii Fe3 Complexes Ions Ligand Substitution Redox Chemical Reactions Principal Oxidation States 2 3 Extraction Gce As A2 Ib A Level Inorganic Chemistry Revision Notes
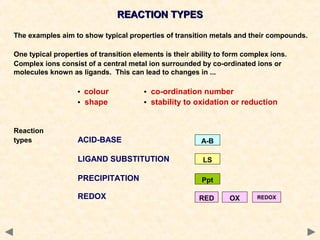
Transition Metal Complexes Unit 1

Hydrated Salts Transition Metals Hexa Aqua Complex Ions Relative Acidity Of Hexaaqua Ions Salt Hydrolysis Why Can Metal Ions Be Acidic A Level Gce As A2 Ib A Level Inorganic Chemistry Revision Notes
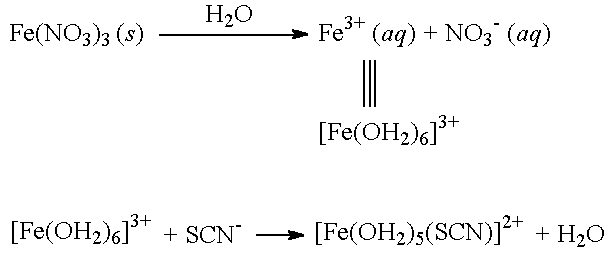
Comments
Post a Comment